Margarine (/ˈmɑːrdʒəriːn/, also UK: /ˈmɑːrɡə-, ˌmɑːrɡəˈriːn, ˌmɑːrdʒə-/, US: /ˈmɑːrdʒərɪn/ )[1] is a spread used for flavoring, baking, and cooking. It is most often used as a substitute for butter. Although originally made from animal fats, most margarine consumed today is made from vegetable oil. The spread was originally named oleomargarine from Latin for oleum (olive oil) and Greek margarite ("pearl", indicating luster). The name was later shortened to margarine.[2]
Margarine consists of a water-in-fat emulsion, with tiny droplets of water dispersed uniformly throughout a fat phase in a stable solid form.[3] While butter is made by concentrating the butterfat of milk through agitation, modern margarine is made through a more intensive processing of refined vegetable oil and water.
Per federal regulation, margarine must have a minimum fat content of 80 percent (with a maximum of 16% water) to be labeled as such in the United States,[4] although the term is used informally to describe vegetable-oil-based spreads with lower fat content.[4][5] In Britain, it can be referred to colloquially as marge.[6]
Margarine can be used as an ingredient in other food products, such as pastries, doughnuts, cakes, and cookies.[7]
Margarine has its roots in the discovery by French chemist Michel Eugène Chevreul in 1813 of margaric acid.[8] Scientists at the time regarded margaric acid, like oleic acid and stearic acid, as one of the three fatty acids that, in combination, form most animal fats. In 1853, the German structural chemist Wilhelm Heinrich Heintz analyzed margaric acid as simply a combination of stearic acid and the previously unknown palmitic acid.[9]
After the French Emperor Napoleon III issued a challenge to create a butter-substitute from beef tallow for the armed forces and lower classes, Hippolyte Mège-Mouriès invented margarine in 1869.[2][10] Mège-Mouriès patented the product, which he named "oleomargarine", and expanded his initial manufacturing operation from France, but had little commercial success. In 1871, he sold the patent to the Dutch company Jurgens, which subsequently became part of Unilever.[2][11] In the same year a German pharmacist, Benedict Klein from Cologne, founded the first margarine factory in Germany, producing the brands Overstolz and Botteram.[12]


The principal raw material in the original formulation of margarine was beef-fat.[2] In 1871, Henry W. Bradley of Binghamton, New York, received U.S. patent 110,626 for a process of creating margarine that combined vegetable oils (primarily cottonseed oil) with animal fats.[13][14] In 1874, the first commercial cargo arrived in the UK. [15] By the late-19th century, some 37 companies were manufacturing margarine in the US, in opposition to the butter industry, which protested and lobbied for government intervention, eventually leading to the 1886 Margarine Act imposing punitive fees against margarine manufacturers.[2]
Shortages in beef-fat supply, combined with advances by James F. Boyce and Paul Sabatier in the hydrogenation of plant materials, soon accelerated the use of Bradley's method, and between 1900 and 1920 commercial oleomargarine was produced from a combination of animal fats and hardened and unhardened vegetable oils.[16] The Great Depression, followed by rationing in the United States and in the United Kingdom, among other countries, during World War II, led to a reduction in supply of animal fat and butter, and, by 1945, "original" margarine had almost completely disappeared from the market.[16] In the United States, problems with supply, coupled with changes in legislation, caused margarine manufacturers to switch almost completely to vegetable oils and fats by 1950, and the margarine industry was ready for an era of product development.[16][dead link]
Carotene in the milk of grass-fed cows gives butter produced from such milk a slightly yellow color. However, being a synthetic product, margarine has a white color resembling lard, which many people found unappetizing. Around the late 1880s, manufacturers began coloring margarine yellow to improve sales.[2]
Dairy firms, especially in Wisconsin, became alarmed at the potential threat to their business, and succeeded in getting legislation passed to prohibit the coloring of the stark white margarine by 1902. In response, margarine companies distributed margarine together with a packet of yellow food coloring.[2] The product was placed in a bowl and the coloring mixed in manually, taking some time and effort, especially if the mixing needed to be done by hand – typically the case at the time since domestic electric mixers were rarely used before the 1920s. It was therefore not unusual for the final product to be served as a light and dark yellow, or even white, striped product. During World War II, there was a shortage of butter in the United States, causing margarine to became popular.[2] In 1951, the W. E. Dennison Company received U.S. patent 2,553,513 for a method to place a capsule of yellow dye inside a plastic package of margarine. After purchase, the capsule was broken by pressing on the outside of the package, and the package was kneaded to distribute the dye.
The artificial coloring laws began being repealed around 1955, and margarine could once again be sold colored like butter in most states. The final hold out was Wisconsin, which finally repealed its restrictions in 1967.[2]
Around the 1930s and 1940s, Arthur Imhausen developed and implemented an industrial process in Germany for producing edible fats by oxidizing synthetic paraffin wax made from coal.[17] The products were fractionally distilled and the edible fats were obtained from the C
9–C
16 fraction[18] which were reacted with glycerol such as that synthesized from propylene.[19] Margarine made from them was found to be nutritious and of agreeable taste, and it was incorporated into diets contributing as much as 700 calories per day.[20][21] The process required at least 60 kilograms of coal per kilogram of synthetic butter.[19] That industrial process was discontinued after WWII due to its inefficiency.
During the Second World War and immediate post-war years amid rationing in the United Kingdom, only two types of margarine were available: a premium brand and a budget brand with whale oil being used in its manufacture.[citation needed] With the end of rationing in 1955, the market was opened to the forces of supply and demand, and brand marketing became prevalent.[16] The competition among the major producers was given further impetus with the beginning of commercial television advertising in 1955 and, throughout the 1950s and 1960s, competing companies vied with each other to produce the margarine that tasted most like butter.[16]
In the mid-1960s, the introduction of two lower-fat blends of butter oil and vegetable oils in Scandinavia, called Lätt & Lagom and Bregott, clouded the issue of what should be called "margarine" and began the debate that led to the introduction of the term "spread".[3] In 1978, an 80% fat product called Krona, made by churning a blend of dairy cream and vegetable oils, was introduced in Europe and, in 1982, a blend of cream and vegetable oils called Clover was introduced in the UK by the Milk Marketing Board.[3] The vegetable oil and cream spread I Can't Believe It's Not Butter! was introduced into the United States in 1981, and in the United Kingdom and Canada in 1991.[22][23][24]
In the 21st century, margarine spreads had many developments to improve their consumer appeal. Most brands phased out the use of hydrogenated oils and became trans fat free. Many brands launched refrigerator-stable margarine spreads that contain only one-third of the fat and calorie content of traditional spreads. Other varieties of spreads include those with added omega-3 fatty acids, low or no salt, added plant sterols (claimed to reduce blood cholesterol), olive oil, or certified vegan oils. In the early 21st century, manufacturers provided margarines in plastic squeeze bottles to ease dispensing and offered pink margarine as a novelty.[2]
.jpg/440px-Swift's_Premium_Oleomargarine_(20107649).jpg)
The basic method of making margarine today consists of emulsifying a blend of oils and fats from vegetable and animal sources, which can be modified using fractionation, interesterification or hydrogenation, with skimmed milk which may be fermented or soured, salt, citric or lactic acid, chilling the mixture to solidify it, and working it to improve the texture.[8][25] Margarines and vegetable fat spreads found in the market can range from 10% to 90% fat, depending on dietary marketing and purpose (spreading, cooking or baking). The softer tub margarines are made with less hydrogenated and more liquid oils than block margarines.[26]
Three types of margarine are common:
To produce margarine, first oils and fats are extracted, e.g. by pressing from seeds, and then refined. Oils may undergo a full or partial hydrogenation process to solidify them. The milk/water mixture is kept separate from the oil mixture until the emulsion step. The fats are warmed so that they are liquid during the mixing process. The water-soluble additives are added to the water or milk mixture, and emulsifiers such as lecithin are added to help disperse the water phase evenly throughout the oil. Other water-soluble additives include powdered skim milk, salt, citric acid, lactic acid, and preservatives such as potassium sorbate. The fat soluble additives are mixed into the oil. These include carotenoids for coloring and antioxidants. Then the two mixtures are emulsified by slowly adding the oil into the milk/water mixture with constant stirring. Next, the mixture is cooled. Rapid chilling avoids the production of large crystals and results in a smooth texture. The product is then rolled or kneaded. Finally, the product may be aerated with nitrogen to facilitate spreading it.
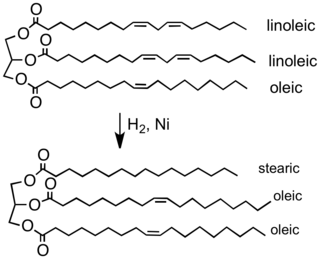
Vegetable and animal fats are similar compounds with different melting points. Fats that are liquid at room temperature are generally known as oils. The melting points are related to the presence of carbon–carbon double bonds in the fatty acids components. A higher number of double bonds gives a lower melting point. Oils can be converted into solid substances at room temperature through hydrogenation.[27]
Commonly, natural oils are hydrogenated by passing hydrogen gas through the oil in the presence of a nickel catalyst, under controlled conditions.[27] The addition of hydrogen to the unsaturated bonds (alkenic double C=C bonds) results in saturated C–C bonds, effectively increasing the melting point of the oil and thus "hardening" it. This is due to the increase in van der Waals' forces between the saturated molecules compared with the unsaturated molecules. However, as there are possible health benefits in limiting the amount of saturated fats in the human diet, the process is controlled so that only enough of the bonds are hydrogenated to give the required texture. Margarines made in this way are said to contain hydrogenated fat.[28] This method is used today for some margarines although the process has been developed and sometimes other metal catalysts are used such as palladium.[8] If hydrogenation is incomplete (partial hardening), the relatively high temperatures used in the hydrogenation process tend to flip some of the carbon–carbon double bonds into the "trans" form. If these particular bonds are not hydrogenated during the process, they remain present in the final margarine in molecules of trans fats,[28] the consumption of which has been shown to be a risk factor for cardiovascular disease.[29] For this reason, partially hardened fats are used less and less in the margarine industry. Some tropical oils, such as palm oil and coconut oil, are naturally semi-solid and do not require hydrogenation.[30][31]