A polytropic process is a thermodynamic process that obeys the relation:
where p is the pressure, V is volume, n is the polytropic index, and C is a constant. The polytropic process equation describes expansion and compression processes which include heat transfer.
Some specific values of n correspond to particular cases:
In addition, when the ideal gas law applies:
Where is the ratio of the heat capacity at constant pressure () to heat capacity at constant volume ().
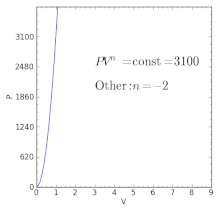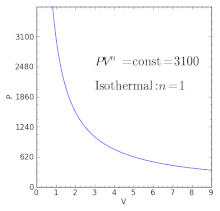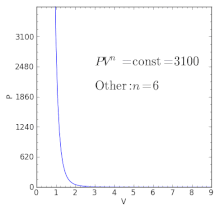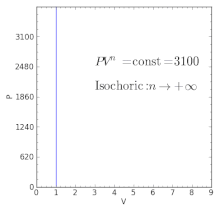
For an ideal gas in a closed system undergoing a slow process with negligible changes in kinetic and potential energy the process is polytropic, such thatwhere C is a constant, , , and with the polytropic coefficient .
For certain values of the polytropic index, the process will be synonymous with other common processes. Some examples of the effects of varying index values are given in the following table.
When the index n is between any two of the former values (0, 1, γ, or ∞), it means that the polytropic curve will cut through (be bounded by) the curves of the two bounding indices.
For an ideal gas, 1 < γ < 5/3, since by Mayer's relation
A solution to the Lane–Emden equation using a polytropic fluid is known as a polytrope.