Barium hydroxide is a chemical compound with the chemical formula Ba(OH)2. The monohydrate (x = 1), known as baryta or baryta-water, is one of the principal compounds of barium. This white granular monohydrate is the usual commercial form.
Barium hydroxide can be prepared by dissolving barium oxide (BaO) in water:
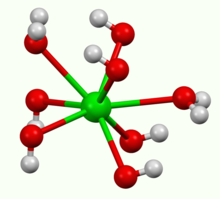
It crystallises as the octahydrate, which converts to the monohydrate upon heating in air. At 100 °C in a vacuum, the monohydrate will yield BaO and water.[3] The monohydrate adopts a layered structure (see picture above). The Ba2+ centers adopt a square antiprismatic geometry. Each Ba2+ center is bound by two water ligands and six hydroxide ligands, which are respectively doubly and triply bridging to neighboring Ba2+ centre sites.[4] In the octahydrate, the individual Ba2+ centers are again eight coordinate but do not share ligands.[5]
Industrially, barium hydroxide is used as the precursor to other barium compounds. The monohydrate is used to dehydrate and remove sulfate from various products.[6] This application exploits the very low solubility of barium sulfate. This industrial application is also applied to laboratory uses.
Barium hydroxide is used in analytical chemistry for the titration of weak acids, particularly organic acids. Its aqueous solution, if clear, is guaranteed to be free of carbonate, unlike those of sodium hydroxide and potassium hydroxide, as barium carbonate is insoluble in water. This allows the use of indicators such as phenolphthalein or thymolphthalein (with alkaline colour changes) without the risk of titration errors due to the presence of carbonate ions, which are much less basic.[7]
Barium hydroxide is occasionally used in organic synthesis as a strong base, for example for the hydrolysis of esters[8] and nitriles,[9][10][11] and as a base in aldol condensations.