The Epstein–Barr virus (EBV), formally called Human gammaherpesvirus 4, is one of the nine known human herpesvirus types in the herpes family, and is one of the most common viruses in humans. EBV is a double-stranded DNA virus.[2] Epstein–Barr virus (EBV) is the first identified oncogenic virus, that is a virus that can cause cancer. EBV establishes permanent infection in humans. It causes infectious mononucleosis and is also tightly linked to many malignant diseases (cancers). Various vaccine formulations underwent testing in different animals or in humans. However, none of them were able to prevent EBV infection and no vaccine has been approved to date.[3]
The virus causes infectious mononucleosis ("mono" or "glandular fever"), a disease characterized by extreme fatigue, fever, sore throat, and swollen lymph nodes. The virus is also associated with various non-malignant, premalignant, and malignant Epstein–Barr virus-associated lymphoproliferative diseases such as Burkitt lymphoma, hemophagocytic lymphohistiocytosis,[4] and Hodgkin's lymphoma; non-lymphoid malignancies such as gastric cancer and nasopharyngeal carcinoma; and conditions associated with human immunodeficiency virus such as hairy leukoplakia and central nervous system lymphomas.[5][6] The virus is also associated with the childhood disorders of Alice in Wonderland syndrome[7] and acute cerebellar ataxia[8] and, by some evidence, higher risks of developing certain autoimmune diseases,[9] especially dermatomyositis, systemic lupus erythematosus, rheumatoid arthritis, and Sjögren's syndrome.[10][11] About 200,000 cancer cases globally per year are thought to be attributable to EBV.[12][13] In 2022, a large study (population of 10 million over 20 years) suggested EBV as the leading cause of multiple sclerosis, with a recent EBV infection causing a 32-fold increase in the risk of developing multiple sclerosis.[14][15][16][17][18]
Infection with EBV occurs by the oral transfer of saliva[19] and genital secretions. Most people become infected with EBV and gain adaptive immunity. In the United States, about half of all five-year-old children and about 90% of adults have evidence of previous infection.[20] Infants become susceptible to EBV as soon as maternal antibody protection disappears. Many children who become infected with EBV display no symptoms or the symptoms are indistinguishable from the other mild, brief illnesses of childhood.[21] When infection occurs during adolescence or young adulthood, it causes infectious mononucleosis 35 to 50% of the time.[22]
EBV infects B cells of the immune system and epithelial cells. Once EBV's initial lytic infection is brought under control, EBV latency persists in the individual's memory B cells for the rest of their life.[19][23][24]
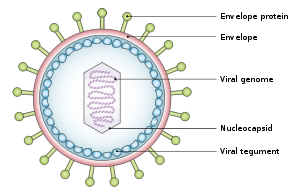
The virus is about 122–180 nm in diameter and is composed of a double helix of deoxyribonucleic acid (DNA) which contains about 172,000 base pairs encoding 85 genes.[19] The DNA is surrounded by a protein nucleocapsid, which is surrounded by a tegument made of protein, which in turn is surrounded by an envelope containing both lipids and surface projections of glycoproteins, which are essential to infection of the host cell.[25] In July 2020, a team of researchers reported the first complete atomic model of the nucleocapsid of the virus. This "first complete atomic model [includes] the icosahedral capsid, the capsid-associated tegument complex (CATC) and the dodecameric portal—the viral genome translocation apparatus."[26][27]
The term viral tropism refers to which cell types that EBV infects. EBV can infect different cell types, including B cells and epithelial cells.[28]
The viral three-part glycoprotein complexes of gHgL gp42 mediate B cell membrane fusion; although the two-part complexes of gHgL mediate epithelial cell membrane fusion. EBV that are made in the B cells have low numbers of gHgLgp42 complexes, because these three-part complexes interact with Human-leukocyte-antigen class II molecules present in B cells in the endoplasmic reticulum and are degraded. In contrast, EBV from epithelial cells are rich in the three-part complexes because these cells do not normally contain HLA class II molecules. As a consequence, EBV made from B cells are more infectious to epithelial cells, and EBV made from epithelial cells are more infectious to B cells. Viruses lacking the gp42 portion are able to bind to human B cells, but unable to infect.[29]

EBV can infect both B cells and epithelial cells. The mechanisms for entering these two cells are different.
To enter B cells, viral glycoprotein gp350 binds to cellular receptor CD21 (also known as CR2).[30] Then, viral glycoprotein gp42 interacts with cellular MHC class II molecules. This triggers fusion of the viral envelope with the cell membrane, allowing EBV to enter the B cell.[25] Human CD35, also known as complement receptor 1 (CR1), is an additional attachment factor for gp350 / 220, and can provide a route for entry of EBV into CD21-negative cells, including immature B-cells. EBV infection downregulates expression of CD35.[31]
To enter epithelial cells, viral protein BMRF-2 interacts with cellular β1 integrins. Then, viral protein gH/gL interacts with cellular αvβ6/αvβ8 integrins. This triggers fusion of the viral envelope with the epithelial cell membrane, allowing EBV to enter the epithelial cell.[25] Unlike B-cell entry, epithelial-cell entry is actually impeded by viral glycoprotein gp42.[30]
Once EBV enters the cell, the viral capsid dissolves and the viral genome is transported to the cell nucleus.[32]
The lytic cycle, or productive infection, results in the production of infectious virions. EBV can undergo lytic replication in both B cells and epithelial cells. In B cells, lytic replication normally only takes place after reactivation from latency. In epithelial cells, lytic replication often directly follows viral entry.[25]
For lytic replication to occur, the viral genome must be linear. The latent EBV genome is circular, so it must linearize in the process of lytic reactivation. During lytic replication, viral DNA polymerase is responsible for copying the viral genome. This contrasts with latency, in which host-cell DNA polymerase copies the viral genome.[25]
Lytic gene products are produced in three consecutive stages: immediate-early, early, and late.[25]Immediate-early lytic gene products act as transactivators, enhancing the expression of later lytic genes. Immediate-early lytic gene products include BZLF1 (also known as Zta, EB1, associated with its product gene ZEBRA) and BRLF1 (associated with its product gene Rta).[25]Early lytic gene products have many more functions, such as replication, metabolism, and blockade of antigen processing. Early lytic gene products include BNLF2.[25]Finally, late lytic gene products tend to be proteins with structural roles, such as VCA, which forms the viral capsid. Other late lytic gene products, such as BCRF1, help EBV evade the immune system.[25]
EGCG, a polyphenol in green tea, has shown in a study to inhibit EBV spontaneous lytic infection at the DNA, gene transcription, and protein levels in a time- and dose-dependent manner; the expression of EBV lytic genes Zta, Rta, and early antigen complex EA-D (induced by Rta), however, the highly stable EBNA-1 gene found across all stages of EBV infection is unaffected.[33] Specific inhibitors (to the pathways) suggest that Ras/MEK/MAPK pathway contributes to EBV lytic infection though BZLF1 and PI3-K pathway through BRLF1, the latter completely abrogating the ability of a BRLF1 adenovirus vector to induce the lytic form of EBV infection.[33] Additionally, the activation of some genes but not others is being studied to determine just how to induce immune destruction of latently infected B cells by use of either TPA or sodium butyrate.[33]

Unlike lytic replication, latency does not result in production of virions.[25]Instead, the EBV genome circular DNA resides in the cell nucleus as an episome and is copied by host-cell DNA polymerase.[25] It persists in the individual's memory B cells.[19][24] Epigenetic changes such as DNA methylation and cellular chromatin constituents, suppress the majority of the viral genes in latently infected cells.[34] Only a portion of EBV's genes are expressed, which support the latent state of the virus.[34][19][35]Latent EBV expresses its genes in one of three patterns, known as latency programs. EBV can latently persist within B cells and epithelial cells, but different latency programs are possible in the two types of cell.[36][37]
EBV can exhibit one of three latency programs: Latency I, Latency II, or Latency III. Each latency program leads to the production of a limited, distinct set of viral proteins and viral RNAs.[38][39]
Also, a program is postulated in which all viral protein expression is shut off (Latency 0).[40]
Within B cells, all three latency programs are possible.[19] EBV latency within B cells usually progresses from Latency III to Latency II to Latency I. Each stage of latency uniquely influences B cell behavior.[19] Upon infecting a resting naïve B cell, EBV enters Latency III. The set of proteins and RNAs produced in Latency III transforms the B cell into a proliferating blast (also known as B cell activation).[19][25] Later, the virus restricts its gene expression and enters Latency II. The more limited set of proteins and RNAs produced in Latency II induces the B cell to differentiate into a memory B cell.[19][25] Finally, EBV restricts gene expression even further and enters Latency I. Expression of EBNA-1 allows the EBV genome to replicate when the memory B cell divides.[19][25]
Within epithelial cells, only Latency II is possible.[41]
In primary infection, EBV replicates in oropharyngeal epithelial cells and establishes Latency III, II, and I infections in B lymphocytes. EBV latent infection of B lymphocytes is necessary for virus persistence, subsequent replication in epithelial cells, and release of infectious virus into saliva. EBV Latency III and II infections of B lymphocytes, Latency II infection of oral epithelial cells, and Latency II infection of NK- or T-cell can result in malignancies, marked by uniform EBV genome presence and gene expression.[42]
Latent EBV in B cells can be reactivated to switch to lytic replication. This is known to happen in vivo, but what triggers it is not known precisely. In vitro, latent EBV in B cells can be reactivated by stimulating the B cell receptor, so it is likely reactivation in vivo takes place after latently infected B cells respond to unrelated infections.[25]
EBV infection of B lymphocytes leads to "immortalization" of these cells, meaning that the virus causes them to continue dividing indefinitely. Normally, cells have a limited lifespan and eventually die, but when EBV infects B lymphocytes, it alters their behavior, making them "immortal" in the sense that they can keep dividing and surviving much longer than usual. This allows the virus to persist in the body for the individual's lifetime.[43]
When EBV infects B cells in vitro, lymphoblastoid cell lines eventually emerge that are capable of indefinite growth. The growth transformation of these cell lines is the consequence of viral protein expression.[44]
EBNA-2, EBNA-3C, and LMP-1, are essential for transformation, whereas EBNA-LP and the EBERs are not.[45]
Following natural infection with EBV, the virus is thought to execute some or all of its repertoire of gene expression programs to establish a persistent infection. Given the initial absence of host immunity, the lytic cycle produces large numbers of virions to infect other (presumably) B-lymphocytes within the host.
The latent programs reprogram and subvert infected B-lymphocytes to proliferate and bring infected cells to the sites at which the virus presumably persists. Eventually, when host immunity develops, the virus persists by turning off most (or possibly all) of its genes and only occasionally reactivates and produces progeny virions. A balance is eventually struck between occasional viral reactivation and host immune surveillance removing cells that activate viral gene expression. The manipulation of the human body's epigenetics by EBV can alter the genome of the cell to leave oncogenic phenotypes.[46] As a result, the modification by the EBV increases the hosts likelihood of developing EBV related cancer.[47] EBV related cancers are unique in that they are frequent to making epigenetic changes but are less likely to mutate.[48]
The site of persistence of EBV may be bone marrow. EBV-positive patients who have had their own bone marrow replaced with bone marrow from an EBV-negative donor are found to be EBV-negative after transplantation.[49]
All EBV nuclear proteins are produced by alternative splicing of a transcript starting at either the Cp or Wp promoters at the left end of the genome (in the conventional nomenclature). The genes are ordered EBNA-LP/EBNA-2/EBNA-3A/EBNA-3B/EBNA-3C/EBNA-1 within the genome.
The initiation codon of the EBNA-LP coding region is created by an alternate splice of the nuclear protein transcript. In the absence of this initiation codon, EBNA-2/EBNA-3A/EBNA-3B/EBNA-3C/EBNA-1 will be expressed depending on which of these genes is alternatively spliced into the transcript.
EBV can be divided into two major types, EBV type 1 and EBV type 2. These two subtypes have different EBNA-3 genes. As a result, the two subtypes differ in their transforming capabilities and reactivation ability. Type 1 is dominant throughout most of the world, but the two types are equally prevalent in Africa. One can distinguish EBV type 1 from EBV type 2 by cutting the viral genome with a restriction enzyme and comparing the resulting digestion patterns by gel electrophoresis.[25]